Chemistry, 01.02.2021 16:40 BigDaddy1220
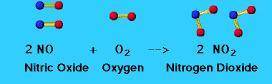
Two volumes of nitric oxide react with one volume of oxygen gas to form two volumes of a reddish-brown gas. Deduce the formula of this gas and sketch particle representations of its molecules.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Two volumes of nitric oxide react with one volume of oxygen gas to form two volumes of a reddish-bro...
Questions

Mathematics, 11.10.2021 22:30


Social Studies, 11.10.2021 22:30

Chemistry, 11.10.2021 22:30

Mathematics, 11.10.2021 22:30



Social Studies, 11.10.2021 22:30

Biology, 11.10.2021 22:30



Arts, 11.10.2021 22:30


English, 11.10.2021 22:30

Biology, 11.10.2021 22:30


Mathematics, 11.10.2021 22:30

English, 11.10.2021 22:30


Mathematics, 11.10.2021 22:30