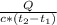Chemistry, 01.02.2021 22:40 ChasityN8491
A quantity of water is heated from 25.0°C to 36.4°C by absorbing 325 J of heat energy. If the specific heat of water is 4.18 J / g°C, what mass is this quantity of water?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
A quantity of water is heated from 25.0°C to 36.4°C by absorbing 325 J of heat energy. If the specif...
Questions




Biology, 20.09.2019 22:00

Mathematics, 20.09.2019 22:00


Mathematics, 20.09.2019 22:00

English, 20.09.2019 22:00



Mathematics, 20.09.2019 22:00


Mathematics, 20.09.2019 22:00





English, 20.09.2019 22:00

Mathematics, 20.09.2019 22:00

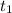 = 25 °C
= 25 °C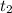 = 36,4 °C
= 36,4 °C )
)