
Chemistry, 02.02.2021 03:10 janellball16
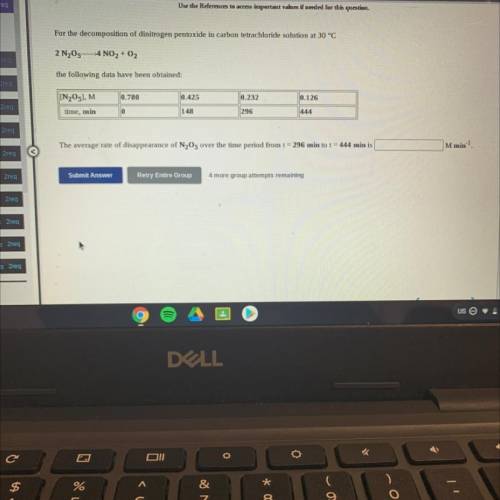
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO2 + O2
the following data have been obtained:
[N205], M
0.780
0.425
0.232
0.126
||444
time, min
0
148
296
M min-1
The average rate of disappearance of N2O5 over the time period from t = 296 min to t = 444 min is

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO...
Questions



Chemistry, 24.10.2021 06:50


Mathematics, 24.10.2021 06:50

Mathematics, 24.10.2021 06:50

Social Studies, 24.10.2021 06:50




Biology, 24.10.2021 06:50





Biology, 24.10.2021 06:50

SAT, 24.10.2021 06:50





