For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rat...

Chemistry, 02.02.2021 03:20 tyneshiajones124
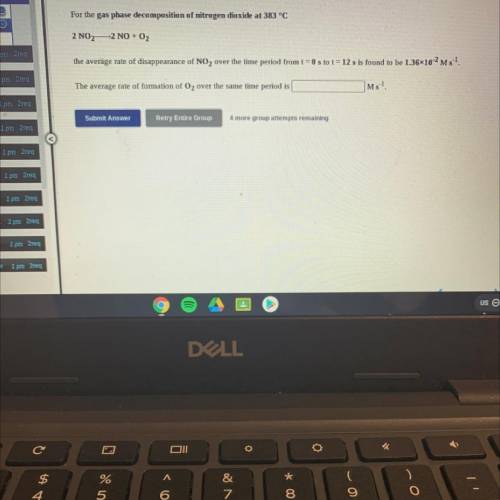
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rate of disappearance of NO2 over the time period from t = 0 s to t = 12 s is found to be 1.3610-2 Ms 1.
The average rate of formation of O2 over the same time period is
Ms-1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Questions

Mathematics, 25.07.2021 23:50








Mathematics, 26.07.2021 01:00


English, 26.07.2021 01:00


English, 26.07.2021 01:00




Mathematics, 26.07.2021 01:00

English, 26.07.2021 01:00




