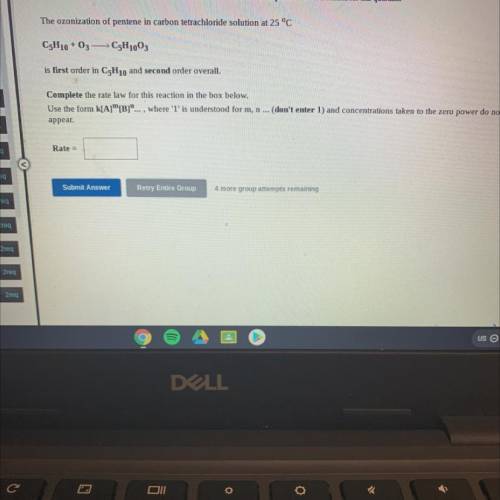
The ozonization of pentene in carbon tetrachloride solution at 25 °C
C5H10 +03 -C5H1003
is fi...

Chemistry, 02.02.2021 03:10 machucagm01
The ozonization of pentene in carbon tetrachloride solution at 25 °C
C5H10 +03 -C5H1003
is first order in C5H10 and second order overall.
Complete the rate law for this reaction in the box below.
Use the form k[A][B]"... , where 'l' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not
appear.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

You know the right answer?
Questions


Mathematics, 31.01.2020 02:43

Chemistry, 31.01.2020 02:43



English, 31.01.2020 02:43


Mathematics, 31.01.2020 02:43

Mathematics, 31.01.2020 02:43



History, 31.01.2020 02:43

Biology, 31.01.2020 02:43

Mathematics, 31.01.2020 02:43

Health, 31.01.2020 02:43

Mathematics, 31.01.2020 02:43


Mathematics, 31.01.2020 02:43




