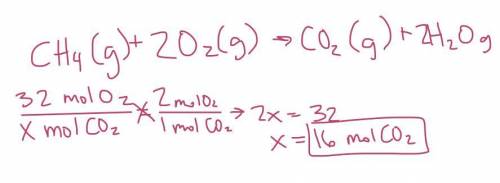Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 22:00
Which sentence best explains the high melting point, boiling point, and surface tension of water? a. the hydrogen atom on each water molecule is strongly attracted to the hydrogen atoms on nearby molecules. b. ions dissolved in the water cause nearby molecules to become temporarily polar. c. the water molecules are connected to each other with covalent bonds. d. water molecules that have lost electrons attract the water molecules that have gained electrons. e. the negative side of each water molecule is attracted to the positive sides of nearby molecules.
Answers: 1
You know the right answer?
How many moles of carbon dioxide are formed when 32 moles of oxygen gas are consumed?
CH4(g) + 2O2(...
Questions


History, 17.07.2020 07:01

Biology, 17.07.2020 07:01


Mathematics, 17.07.2020 07:01

Health, 17.07.2020 07:01




Mathematics, 17.07.2020 07:01

English, 17.07.2020 07:01

Mathematics, 17.07.2020 07:01

Spanish, 17.07.2020 07:01


English, 17.07.2020 07:01

Mathematics, 17.07.2020 07:01



Mathematics, 17.07.2020 07:01

Advanced Placement (AP), 17.07.2020 07:01