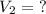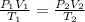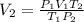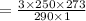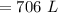Chemistry, 02.02.2021 14:00 seider7997
A gas has a volume of 250.0 liters at 17.0 °C and 3.00 atm of pressure. what volume must the gas be increased for the gas to be at STP conditions?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
A gas has a volume of 250.0 liters at 17.0 °C and 3.00 atm of pressure. what volume must the gas be...
Questions

English, 29.03.2020 22:33


Mathematics, 29.03.2020 22:33




Mathematics, 29.03.2020 22:33

Mathematics, 29.03.2020 22:33


Mathematics, 29.03.2020 22:34

Mathematics, 29.03.2020 22:34

Spanish, 29.03.2020 22:34

Physics, 29.03.2020 22:34

Mathematics, 29.03.2020 22:34

Mathematics, 29.03.2020 22:34

Mathematics, 29.03.2020 22:34

English, 29.03.2020 22:34



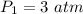 Final,
Final, 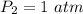
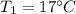 or,
or, 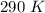 Final,
Final, 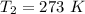
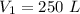 Final,
Final,