
Chemistry, 02.02.2021 16:50 daeshawnc14
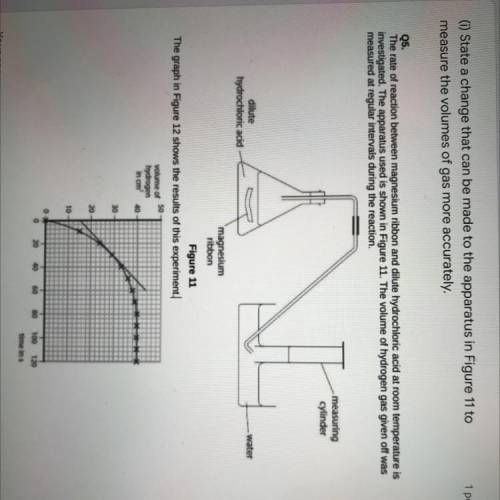
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more accurately.
Q5.
The rate of reaction between magnesium ribbon and dilute hydrochloric acid at room temperature is
investigated. The apparatus used is shown in Figure 11. The volume of hydrogen gas given off was
measured at regular intervals during the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more...
Questions


History, 04.07.2019 23:00

Chemistry, 04.07.2019 23:00



Mathematics, 04.07.2019 23:00

History, 04.07.2019 23:00



Mathematics, 04.07.2019 23:00

Social Studies, 04.07.2019 23:00

History, 04.07.2019 23:00



Mathematics, 04.07.2019 23:00


Mathematics, 04.07.2019 23:00


English, 04.07.2019 23:00



