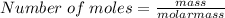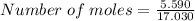Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

You know the right answer?
Calculate the moles of ammonia present in a 5.590 g sample if the molar mass of ammonia is 17.030 g/...
Questions


Chemistry, 27.09.2019 18:50


Physics, 27.09.2019 18:50

Mathematics, 27.09.2019 18:50

Social Studies, 27.09.2019 18:50

Biology, 27.09.2019 18:50



History, 27.09.2019 18:50

History, 27.09.2019 18:50

Social Studies, 27.09.2019 18:50


Geography, 27.09.2019 18:50

Computers and Technology, 27.09.2019 18:50



Mathematics, 27.09.2019 18:50