Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
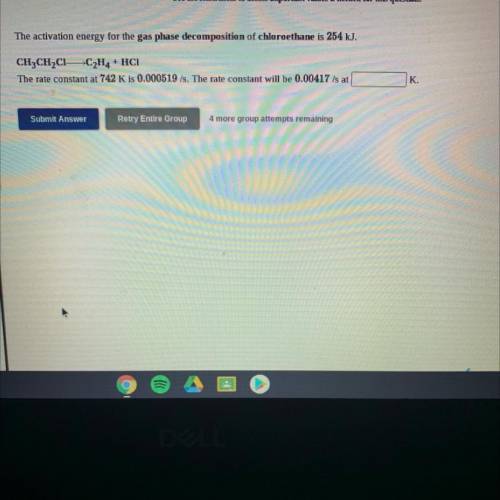
The activation energy for the gas phase decomposition of chloroethane is 254 kJ.
CH3CH2C1-C2H4 + HC...
Questions


English, 02.04.2020 10:41


Mathematics, 02.04.2020 10:41

Mathematics, 02.04.2020 10:41

Mathematics, 02.04.2020 10:41




Health, 02.04.2020 10:42

Mathematics, 02.04.2020 10:42

Mathematics, 02.04.2020 10:42


Mathematics, 02.04.2020 10:43

Mathematics, 02.04.2020 10:43


English, 02.04.2020 10:43

Business, 02.04.2020 10:44

Mathematics, 02.04.2020 10:45