
Chemistry, 03.02.2021 20:00 sunshine52577oyeor9
During the reaction P4 + 5O 2 P4 O10, 1.5 moles of product was made in 30 seconds. What is the rate of reaction?
a) 0.011 g/min
b) 210 g/min
c) 380 g/min
d) 850 g/min

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
During the reaction P4 + 5O 2 P4 O10, 1.5 moles of product was made in 30 seconds. What is the rate...
Questions


Physics, 16.12.2020 06:50

Mathematics, 16.12.2020 06:50

Physics, 16.12.2020 06:50

Mathematics, 16.12.2020 06:50


English, 16.12.2020 06:50


Mathematics, 16.12.2020 07:00



Mathematics, 16.12.2020 07:00


Mathematics, 16.12.2020 07:00




Mathematics, 16.12.2020 07:00


English, 16.12.2020 07:00

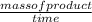 =
= 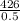 = 850g/min
= 850g/min

