
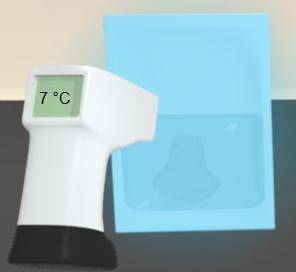
For the cold pack shown below, the temperature on the outside surface has dropped considerably from its initial temperature of 25 °C. Which of the following provides the best explanation for this temperature decrease?
A. The cold pack is releasing cold to the surroundings.
B. Ice is beginning to form on the outside of the bag, lowering its temperature.
C. Energy is traveling out of the bag into the surroundings, making the bag colder.
D. The thermometer is measuring the temperature of the surroundings, which is losing energy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
For the cold pack shown below, the temperature on the outside surface has dropped considerably from...
Questions

Mathematics, 03.12.2020 02:10


Medicine, 03.12.2020 02:10


History, 03.12.2020 02:10


Mathematics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10



Mathematics, 03.12.2020 02:10


History, 03.12.2020 02:10

English, 03.12.2020 02:10

English, 03.12.2020 02:10

Business, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

History, 03.12.2020 02:10


English, 03.12.2020 02:10



