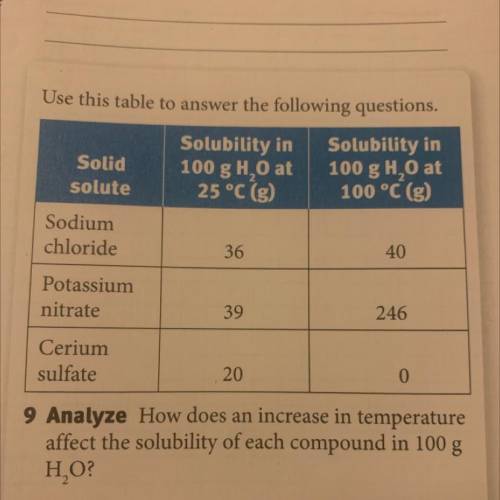
How does an increase in temperature affect the solubility of each compound in 100 g H2O?
...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Questions

Social Studies, 21.08.2019 22:00


Health, 21.08.2019 22:00


Physics, 21.08.2019 22:00

English, 21.08.2019 22:00


Computers and Technology, 21.08.2019 22:00


Biology, 21.08.2019 22:00

Mathematics, 21.08.2019 22:00


History, 21.08.2019 22:00

Mathematics, 21.08.2019 22:00



Mathematics, 21.08.2019 22:00


Mathematics, 21.08.2019 22:00

Computers and Technology, 21.08.2019 22:00