
Chemistry, 04.02.2021 14:00 bethanybowers4986
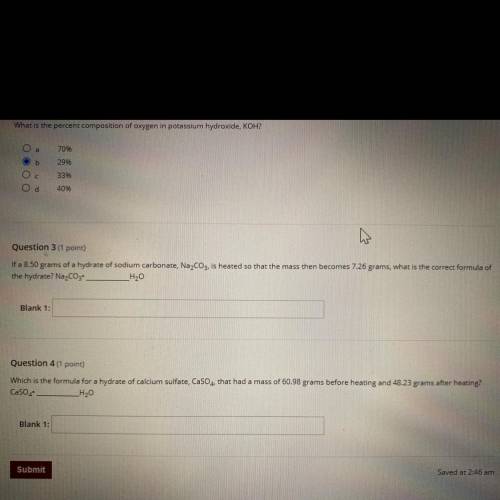
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that the mass then becomes 7.26 grams, what is the correct formula of
the hydrate? Na2CO3 _H20
Blank 1:
Question 4 (1 point)
Which is the formula for a hydrate of calcium sulfate, CaSO4, that had a mass of 60.98 grams before heating and 48.23 grams after heating?
CaSO4
_H20
Blank 1:

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that th...
Questions

Mathematics, 18.05.2021 09:10


Mathematics, 18.05.2021 09:10

English, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10



Mathematics, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10


World Languages, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10

Mathematics, 18.05.2021 09:10

History, 18.05.2021 09:10


Mathematics, 18.05.2021 09:10

English, 18.05.2021 09:10



