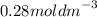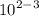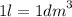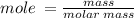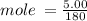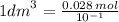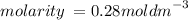Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Calculate the molarity of a solution made by dissolving 5.00 g of glucose (C6H12O6) in sufficient wa...
Questions

Mathematics, 11.03.2020 01:07


History, 11.03.2020 01:07

Mathematics, 11.03.2020 01:07

Mathematics, 11.03.2020 01:07

Mathematics, 11.03.2020 01:07






Computers and Technology, 11.03.2020 01:08




Mathematics, 11.03.2020 01:08