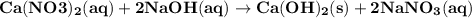Chemistry, 05.02.2021 04:00 ijeomaokoro2264
For the reaction between aqueous calcium nitrate and aqueous sodium hydroxide producing the precipitate (solid) calcium hydroxide and aqueous sodium nitrate
(a) write a balanced equation for this reaction
(b) classify the reaction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
For the reaction between aqueous calcium nitrate and aqueous sodium hydroxide producing the precipit...
Questions



Mathematics, 22.01.2021 21:30


English, 22.01.2021 21:30


Mathematics, 22.01.2021 21:30

History, 22.01.2021 21:30




Mathematics, 22.01.2021 21:30



Mathematics, 22.01.2021 21:30

Mathematics, 22.01.2021 21:30

Mathematics, 22.01.2021 21:30

Mathematics, 22.01.2021 21:30

History, 22.01.2021 21:30

Mathematics, 22.01.2021 21:30