Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

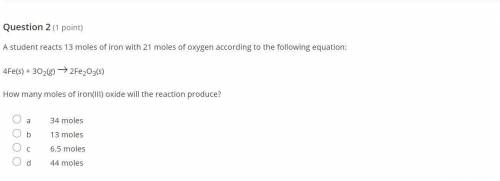
You know the right answer?
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
4Fe(...
Questions

Mathematics, 09.04.2021 02:00


Biology, 09.04.2021 02:00

Social Studies, 09.04.2021 02:00

Mathematics, 09.04.2021 02:00


Mathematics, 09.04.2021 02:00

Social Studies, 09.04.2021 02:00




History, 09.04.2021 02:00

Mathematics, 09.04.2021 02:00

Mathematics, 09.04.2021 02:00

Computers and Technology, 09.04.2021 02:00



History, 09.04.2021 02:00