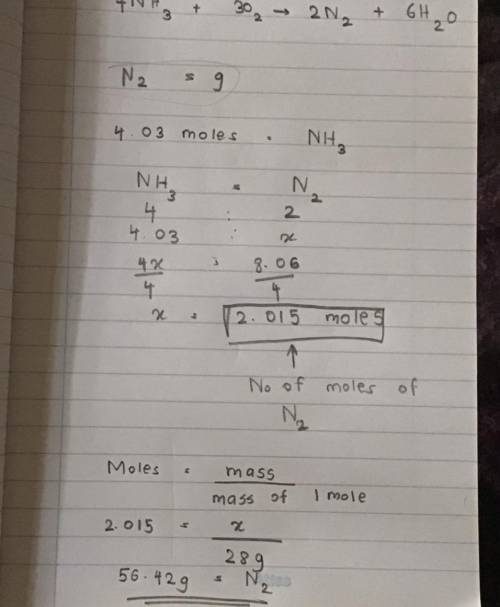Using the balanced chemical equation:
4NH3 + 302 --> 2N2 + 6H20
Determine the amount of gr...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Questions

Mathematics, 19.11.2020 09:20


Arts, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

History, 19.11.2020 09:20

Biology, 19.11.2020 09:20

History, 19.11.2020 09:20

English, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20


Biology, 19.11.2020 09:20

Computers and Technology, 19.11.2020 09:20

Arts, 19.11.2020 09:20


Physics, 19.11.2020 09:20

Physics, 19.11.2020 09:20

English, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20