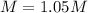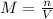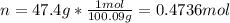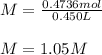Chemistry, 05.02.2021 09:20 BIKRAMlawati9684
47.4 grams of calcium carbonate (CaCO3) is dissolved in 450 mL of water. What is the molarity of this aqueous solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
47.4 grams of calcium carbonate (CaCO3) is dissolved in 450 mL of water. What is the molarity of thi...
Questions

Mathematics, 27.04.2021 01:00

Chemistry, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

English, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


History, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Physics, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00




Law, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00