Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
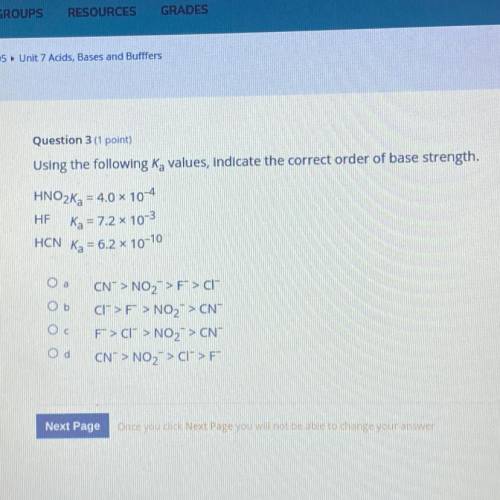
Using the following Ka values, indicate the correct order of base strength.
HNO2Ka = 4.0 x 10-4
Questions


Mathematics, 08.11.2020 21:00

Mathematics, 08.11.2020 21:00

Chemistry, 08.11.2020 21:00

Mathematics, 08.11.2020 21:00


History, 08.11.2020 21:00


Mathematics, 08.11.2020 21:00

Physics, 08.11.2020 21:00

English, 08.11.2020 21:00


English, 08.11.2020 21:00

Physics, 08.11.2020 21:00


Mathematics, 08.11.2020 21:00

Social Studies, 08.11.2020 21:00

Physics, 08.11.2020 21:00

Mathematics, 08.11.2020 21:00

Biology, 08.11.2020 21:00