Can someone please help me
For the balanced equation shown below,
how many grams of KNO3 (mm=...

Chemistry, 05.02.2021 19:10 emarquez05
Can someone please help me
For the balanced equation shown below,
how many grams of KNO3 (mm=101.1)
reacted, if 1.26 moles of 02 (mm= 32)
are produced? 2KNO3=>2KNO2+02
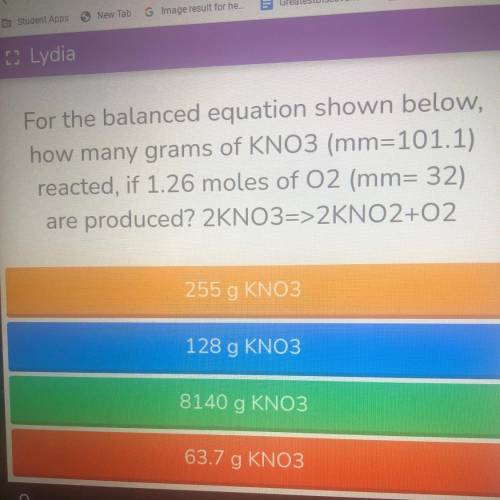

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Questions


Biology, 05.02.2020 11:00


Social Studies, 05.02.2020 11:01

Mathematics, 05.02.2020 11:01

Mathematics, 05.02.2020 11:01

Mathematics, 05.02.2020 11:01




Mathematics, 05.02.2020 11:01





Mathematics, 05.02.2020 11:01

Physics, 05.02.2020 11:01


English, 05.02.2020 11:01



