
Chemistry, 05.02.2021 21:30 donttrip10
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of water produced by the reaction of of oxygen.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

You know the right answer?
Acetylene gas is often used in welding torches because of the very high heat produced when it reacts...
Questions

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20



Social Studies, 15.01.2021 21:20


Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20





Advanced Placement (AP), 15.01.2021 21:20


Mathematics, 15.01.2021 21:20

Mathematics, 15.01.2021 21:20

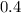 moles of water
moles of water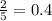 moles of water
moles of water

