What is the best reason for the difference in properties of licl and c6h14o?
licl and c6h14o...

Chemistry, 06.10.2019 21:00 raquelqueengucci25
What is the best reason for the difference in properties of licl and c6h14o?
licl and c6h14o have different atomic masses.
cl and o belong to different groups of the periodic table.
one compound is ionic, and the other is metallic.
one compound is covalent, and the other is ionic.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Questions

Mathematics, 07.11.2020 01:00

Arts, 07.11.2020 01:00

Health, 07.11.2020 01:00



History, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00


Arts, 07.11.2020 01:00


Mathematics, 07.11.2020 01:00

Biology, 07.11.2020 01:00

English, 07.11.2020 01:00

Arts, 07.11.2020 01:00

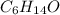 is a organic compound formed by the sharing of electrons between the elements, hence is termed as a covalent compound.
is a organic compound formed by the sharing of electrons between the elements, hence is termed as a covalent compound.

