

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
Some antacid tables contain aluminum hydroxide. The aluminum hydroxide reacts with stomach acid acco...
Questions

Mathematics, 09.05.2021 06:50

Social Studies, 09.05.2021 06:50


English, 09.05.2021 06:50

Mathematics, 09.05.2021 06:50



Mathematics, 09.05.2021 06:50


Computers and Technology, 09.05.2021 06:50

Social Studies, 09.05.2021 06:50


History, 09.05.2021 06:50


Mathematics, 09.05.2021 06:50

Mathematics, 09.05.2021 06:50



Physics, 09.05.2021 07:00

History, 09.05.2021 07:00

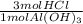 = 26.67 mol HCl
= 26.67 mol HCl

