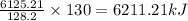Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

You know the right answer?
The standard heat of combustion is shown in the following chemical equation CgH 20 (g) + 140 2(g) 9C...
Questions

History, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

History, 29.01.2021 03:00

Chemistry, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00


Mathematics, 29.01.2021 03:00



English, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00


Biology, 29.01.2021 03:00


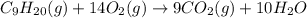
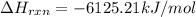 . If 130 g of nonane combusts , how much heat is released?
. If 130 g of nonane combusts , how much heat is released?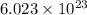 of particles.
of particles.
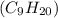 weighs = 128.2 g
weighs = 128.2 g 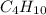 on combustion releases =
on combustion releases =