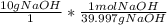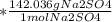Chemistry, 08.02.2021 02:30 ariellake8551
(05.04 MC)
The following reaction shows sodium hydroxide reacting with sulfuric acid.
NaOH + H2SO4 → Na2SO4 + H2O
How many grams of Na2SO4 are produced from 10.0 grams of NaOH?
(Molar mass of Na = 22.989 g/mol, O = 15.999 g/mol, H = 1.008 g/mol, S = 32.065 g/mol) (4 points)
a
17.8 grams
b
19.2 grams
c
35.5 grams
d
38.5 grams
will mark brainliest

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
(05.04 MC)
The following reaction shows sodium hydroxide reacting with sulfuric acid.
N...
N...
Questions



Mathematics, 22.05.2020 08:05


Business, 22.05.2020 08:05




Mathematics, 22.05.2020 08:05


Mathematics, 22.05.2020 08:05


Mathematics, 22.05.2020 08:05


Mathematics, 22.05.2020 08:05


Mathematics, 22.05.2020 08:05


Mathematics, 22.05.2020 08:05