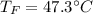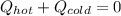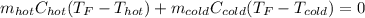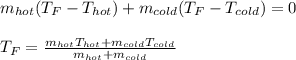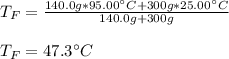Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
If you combine 300.0 mL of water at 25.00 ∘C and 140.0 mL of water at 95.00 ∘C, what is the final te...
Questions

Spanish, 30.09.2020 05:01



Mathematics, 30.09.2020 05:01





English, 30.09.2020 05:01

Physics, 30.09.2020 05:01





Medicine, 30.09.2020 05:01



Engineering, 30.09.2020 05:01

Mathematics, 30.09.2020 05:01