Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
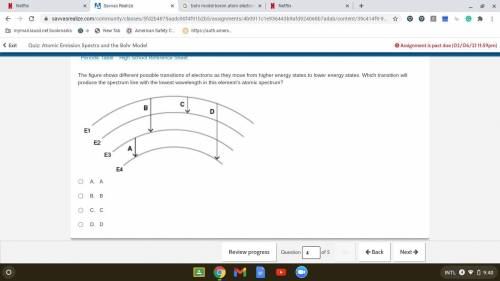
The figure shows different possible transitions of electrons as they move from higher energy states...
Questions

Mathematics, 24.05.2020 06:01







English, 24.05.2020 06:01





Mathematics, 24.05.2020 06:01





Mathematics, 24.05.2020 06:01

Mathematics, 24.05.2020 06:01

Mathematics, 24.05.2020 06:01