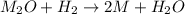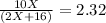Chemistry, 08.02.2021 14:00 Nickanderson21
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide? (Use molar masses)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal...
Questions


Mathematics, 07.04.2020 19:38


Mathematics, 07.04.2020 19:38


Mathematics, 07.04.2020 19:38



Mathematics, 07.04.2020 19:38









Biology, 07.04.2020 19:38


English, 07.04.2020 19:38