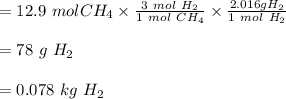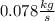The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°C and 0.96atm. Calculate the rate at which dihydrogen is being produced.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of c...
Questions



History, 31.08.2021 19:40

Mathematics, 31.08.2021 19:40


Health, 31.08.2021 19:40

English, 31.08.2021 19:40


Mathematics, 31.08.2021 19:40


Biology, 31.08.2021 19:50



Mathematics, 31.08.2021 19:50

Biology, 31.08.2021 19:50

Mathematics, 31.08.2021 19:50


Mathematics, 31.08.2021 19:50

Computers and Technology, 31.08.2021 19:50

Mathematics, 31.08.2021 19:50

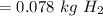 ".
".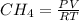

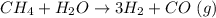
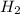 produced:
produced: