
Chemistry, 08.02.2021 19:10 vondah4014
Sam records the mass of his evaporating dish as 6.488 g. He records the mass of the evaporating dish and the sample of hydrate as 19.72 g. After heating the sample in the evaporating dish to constant weight, the mass of them combined is 11.344 g. How many moles of water were removed from the sample by the heating process?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Sam records the mass of his evaporating dish as 6.488 g. He records the mass of the evaporating dish...
Questions







Mathematics, 08.07.2020 17:01

Biology, 08.07.2020 17:01











Computers and Technology, 08.07.2020 17:01

Mathematics, 08.07.2020 17:01

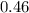 moles of water were removed from the sample by the heating process
moles of water were removed from the sample by the heating process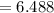 grams
grams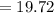 grams
grams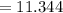 grams
grams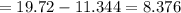 grams
grams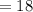 grams
grams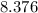 Grams of water is equal to
Grams of water is equal to 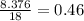 moles
moles

