
Chemistry, 08.02.2021 20:30 SophieStar15
ANSWER ASAP
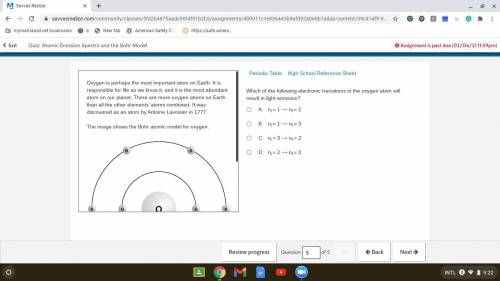
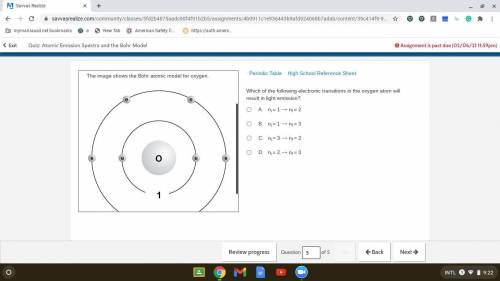
Which of the following electronic transitions in the oxygen atom will result in light emission?
. A. ni = 1 ⟶ nf = 2
B. ni = 1 ⟶ nf = 3
C. ni = 3 ⟶ nf = 2
D. ni = 2 ⟶ nf = 3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

You know the right answer?
ANSWER ASAP
Which of the following electronic transitions in the oxygen atom will result in light e...
Questions

Mathematics, 04.05.2021 22:30

History, 04.05.2021 22:30

Biology, 04.05.2021 22:30

Mathematics, 04.05.2021 22:30



Mathematics, 04.05.2021 22:30

English, 04.05.2021 22:30


English, 04.05.2021 22:30





Advanced Placement (AP), 04.05.2021 22:30

Arts, 04.05.2021 22:30

Mathematics, 04.05.2021 22:30

Mathematics, 04.05.2021 22:30




