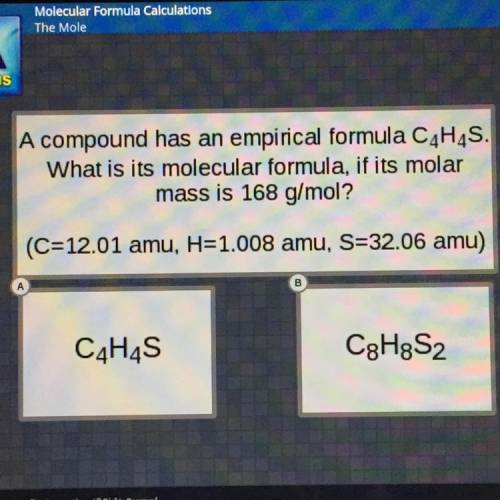
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass i...

Chemistry, 09.02.2021 01:00 natalyarenassalgado
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass is 168 g/mol?
(C=12.01 amu, H=1.008 amu, S=32.06 amu)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
Questions




History, 13.05.2021 23:00

Arts, 13.05.2021 23:00






English, 13.05.2021 23:00



Social Studies, 13.05.2021 23:00

Social Studies, 13.05.2021 23:00

Biology, 13.05.2021 23:00


Physics, 13.05.2021 23:00

Computers and Technology, 13.05.2021 23:00



