Chemistry, 09.02.2021 01:00 thomasmurphy200
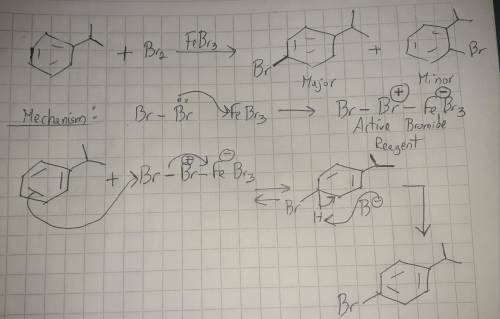
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropylbenzene. Complete the curved-arrow mechanism below, beginning with formation of the active brominating reagent. Remember to include lone pairs and formal charges where appropriate.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropy...
Questions

History, 13.01.2021 05:00

Biology, 13.01.2021 05:00

Mathematics, 13.01.2021 05:00


English, 13.01.2021 05:00

Arts, 13.01.2021 05:00

History, 13.01.2021 05:00




Mathematics, 13.01.2021 05:00

Physics, 13.01.2021 05:00

Arts, 13.01.2021 05:00


Mathematics, 13.01.2021 05:00


Mathematics, 13.01.2021 05:00