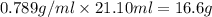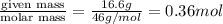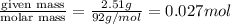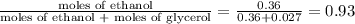Glycerol (C3H8O3), also called glycerine, is widely used in the food and pharmaceutical industries. Glycerol is polar and dissolves readily in water and polar organic solvents like ethanol. Calculate the mole fraction of the solvent in a solution that contains 2.51 g glycerol dissolved in 21.10 mL ethanol (CH3CH2OH; density

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Glycerol (C3H8O3), also called glycerine, is widely used in the food and pharmaceutical industries....
Questions

Mathematics, 11.02.2021 22:30


Mathematics, 11.02.2021 22:30


Biology, 11.02.2021 22:30

Business, 11.02.2021 22:30


Mathematics, 11.02.2021 22:30

History, 11.02.2021 22:30

Computers and Technology, 11.02.2021 22:30





English, 11.02.2021 22:30

Mathematics, 11.02.2021 22:30

Chemistry, 11.02.2021 22:30


Mathematics, 11.02.2021 22:30

Mathematics, 11.02.2021 22:30