40 POINTS!
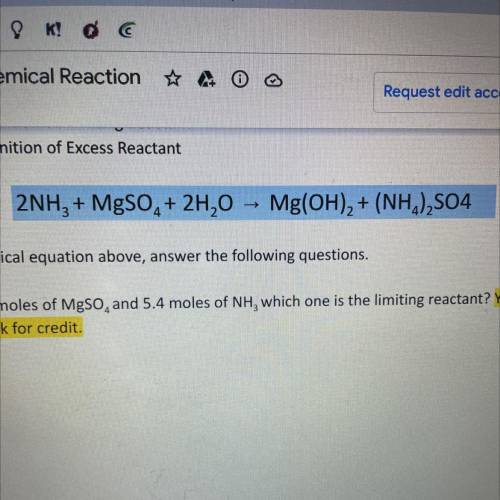
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation ab...

Chemistry, 09.02.2021 01:30 emalvidrez5205
40 POINTS!
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation above answer the following questions. SHOW ALL WORK OR RECEIVE NO CREDIT
A. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting reactant? Show work for credit
B. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of MgSO4 and 5.4 moles of NH3? Show work for credit
C. How many moles of the excess reactant is left over after the reaction has been completed? Show work for credit

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Questions

Mathematics, 03.06.2021 22:50



Mathematics, 03.06.2021 22:50


Mathematics, 03.06.2021 22:50


Biology, 03.06.2021 22:50

Mathematics, 03.06.2021 22:50



Health, 03.06.2021 22:50




History, 03.06.2021 22:50

Chemistry, 03.06.2021 22:50

Computers and Technology, 03.06.2021 22:50




