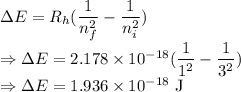Chemistry, 09.02.2021 02:10 hunterbetterton1
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition from =3 to =1.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition...
Questions

Mathematics, 31.03.2021 21:50


Mathematics, 31.03.2021 21:50

Chemistry, 31.03.2021 21:50

English, 31.03.2021 21:50



Mathematics, 31.03.2021 21:50

Mathematics, 31.03.2021 21:50




Mathematics, 31.03.2021 21:50




Physics, 31.03.2021 21:50


Mathematics, 31.03.2021 21:50

Social Studies, 31.03.2021 21:50

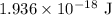
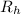 = Rydberg constant =
= Rydberg constant = 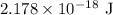
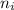 = Initial shell = 3
= Initial shell = 3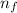 = Final shell = 1
= Final shell = 1