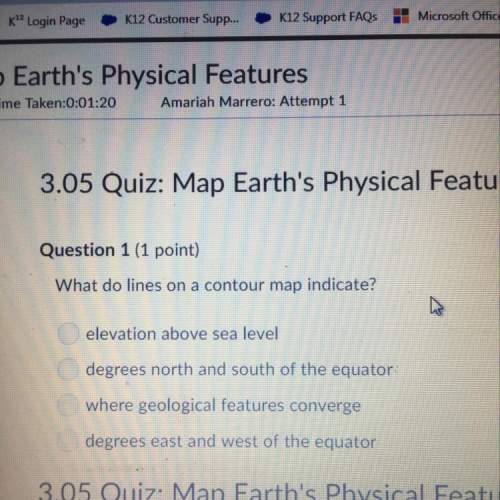
Chemistry, 09.02.2021 04:10 vicky10445
Phosgene (Cl2CO), a poison gas used in World War I , is formed by the reaction of Cl2 and CO. The proposed mechanism for the reaction is
Cl2⇌2Cl(fast, equilibrium)
Cl+CO⇌ClCO(fast, equilibrium)
ClCO+Cl2→Cl2CO+Cl(slow)
What rate law is consistent with this mechanism?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
Phosgene (Cl2CO), a poison gas used in World War I , is formed by the reaction of Cl2 and CO. The pr...
Questions



Mathematics, 09.11.2019 05:31

Biology, 09.11.2019 05:31

Physics, 09.11.2019 05:31

Mathematics, 09.11.2019 05:31

Biology, 09.11.2019 05:31

Mathematics, 09.11.2019 05:31

History, 09.11.2019 05:31


Health, 09.11.2019 05:31



Biology, 09.11.2019 05:31




Mathematics, 09.11.2019 05:31

History, 09.11.2019 05:31

Health, 09.11.2019 05:31