Consider the equations below
A
CH (9) →C(s)+ 2H2(9) AH= 74,6 kJ
C(s) +2012(g) → CCI,(9)...

Chemistry, 09.02.2021 06:00 rhaquan66766
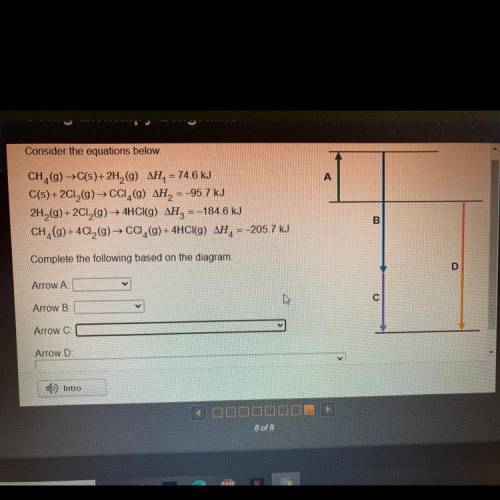
Consider the equations below
A
CH (9) →C(s)+ 2H2(9) AH= 74,6 kJ
C(s) +2012(g) → CCI,(9) AH = -95.7 kJ
2H2(g) +2012(9) — 4HCl(g) AH, =-184.6 kJ
CH_(9)+ 4C12(g) → CC,(g) + 4HCI(g) AH= -205,7 kJ
B
Complete the following based on the diagram
D
Arrow A
✓
Arrow B
no
с
Arrow C
Arrow D

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
Questions



History, 20.09.2020 14:01

History, 20.09.2020 14:01




English, 20.09.2020 14:01

History, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01






Chemistry, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

SAT, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01



