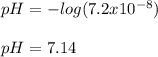Chemistry, 09.02.2021 07:50 tyryceschnitker
Calculate the ph of a solution with a [H 3 0+]=7.2×10-8 M

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Calculate the ph of a solution with a [H 3 0+]=7.2×10-8 M...
Questions




English, 09.10.2019 20:20


Geography, 09.10.2019 20:20

History, 09.10.2019 20:20


German, 09.10.2019 20:20



Biology, 09.10.2019 20:20



English, 09.10.2019 20:20

History, 09.10.2019 20:20

History, 09.10.2019 20:20



![pH=-log([H_3O^+])](/tpl/images/1104/1503/26c02.png)