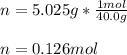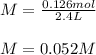Chemistry, 09.02.2021 08:20 urfavgringo
If 5.025 grams of NaOH are dissolved in 2.4 liters of water, what is the Molarity of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
If 5.025 grams of NaOH are dissolved in 2.4 liters of water, what is the Molarity of the solution?...
Questions

English, 22.06.2021 02:40



Computers and Technology, 22.06.2021 02:40



Mathematics, 22.06.2021 02:40


Chemistry, 22.06.2021 02:40

Mathematics, 22.06.2021 02:40








Mathematics, 22.06.2021 02:40

Mathematics, 22.06.2021 02:40