
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganate. They react according to the following equation
KMnO4 + K2C2O4 → K2Mn + 2CO2 + 202
If the reaction has a 53% yield, how many grams of carbon dioxide does it produce?
А
11.79
B
44.09
23.39
2209
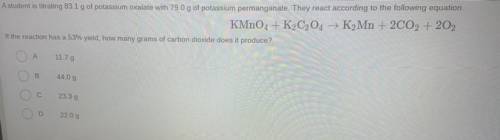

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganat...
Questions


Social Studies, 19.01.2020 07:31


Geography, 19.01.2020 07:31


Biology, 19.01.2020 07:31

History, 19.01.2020 07:31

Physics, 19.01.2020 07:31

Mathematics, 19.01.2020 07:31

History, 19.01.2020 07:31





English, 19.01.2020 07:31

Mathematics, 19.01.2020 07:31

Social Studies, 19.01.2020 07:31


Mathematics, 19.01.2020 07:31



