
Chemistry, 10.02.2021 19:20 bobduncan1086
What is the molarity of the solution of Sodium hydroxide, if 35 ml of 0.1 M HCl is used in the titration of 25 ml of the barium hydroxide solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
What is the molarity of the solution of Sodium hydroxide, if 35 ml of 0.1 M HCl is used in the titra...
Questions

Mathematics, 05.05.2020 17:40

Biology, 05.05.2020 17:40

English, 05.05.2020 17:40

Biology, 05.05.2020 17:40

English, 05.05.2020 17:40







Computers and Technology, 05.05.2020 17:40



Mathematics, 05.05.2020 17:40





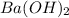 is 0.07 M
is 0.07 M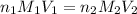
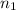 = basicity
= basicity 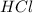 = 1
= 1
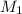 = molarity of
= molarity of 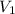 = volume of
= volume of 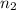 = acidity of
= acidity of 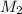 = molarity of
= molarity of 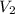 = volume of
= volume of 



