Use for questions 1-4
Zn + 2HCl → ZnCl2 + H2
1) If 0.8 moles of Zn are used,
how...

Chemistry, 10.02.2021 20:50 NikolaiSolokov
Use for questions 1-4
Zn + 2HCl → ZnCl2 + H2
1) If 0.8 moles of Zn are used,
how many moles of HCl are
required?
2) If 3.5 moles of Zn are used,
how many moles of HCl are
required?
3) If 4.8 moles of HCl are used,
how many moles of H, are
produced?
4) If 0.48 moles of ZnCl, are
produced, how many moles of
HCl are needed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1
You know the right answer?
Questions

Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57


Mathematics, 05.06.2020 17:57



Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57






Mathematics, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

Arts, 05.06.2020 17:57

Mathematics, 05.06.2020 17:57

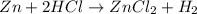
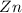 require = 2 moles of
require = 2 moles of 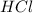
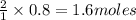 of
of 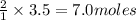 of
of 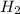
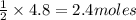 of
of 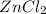 are produced by = 2 moles of
are produced by = 2 moles of 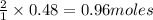 of
of 

