Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
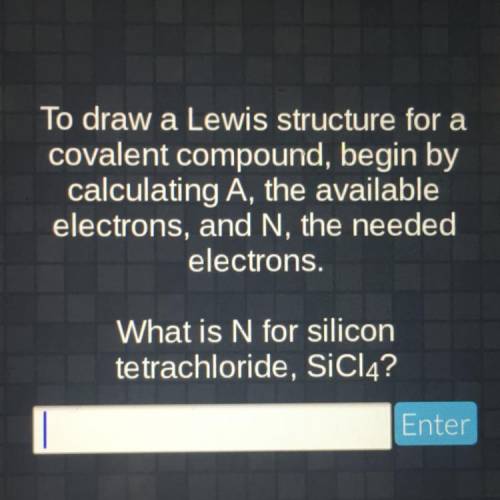
To draw a Lewis structure for a covalent compound, begin by calculating A, the available electrons,...
Questions

Mathematics, 20.11.2020 01:20

Health, 20.11.2020 01:20

Advanced Placement (AP), 20.11.2020 01:20

Biology, 20.11.2020 01:20



Biology, 20.11.2020 01:20




Mathematics, 20.11.2020 01:20


History, 20.11.2020 01:20


Mathematics, 20.11.2020 01:20


Mathematics, 20.11.2020 01:20

Mathematics, 20.11.2020 01:20


Mathematics, 20.11.2020 01:20