
Chemistry, 11.02.2021 02:10 blessednish86orogbi
Answer questions 1-6 using the information below:
2 A(aq) + 3 B(aq) LaTeX: \longrightarrow⟶ A2B3(aq)
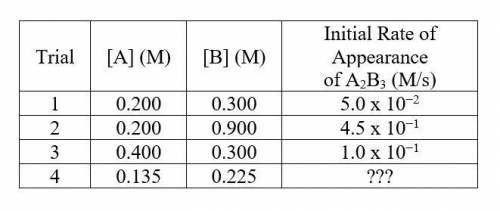
An experiment is conducted at 25 C and the rate of appearance of the product A2B3(aq) is measured as the concentrations of the reactants were varied. Data from the trials are shown below: -picture-
1. Determine the rate law for the reaction at 25 C. Justify your answer.
2. Determine the value of k, including units.
3. Determine the rate of trial 4.
4. A possible reaction mechanism has a 1st elementary step as shown below:
A + B ⟶ AB
Could this first step be the rate determining step? Explain your reasoning.
5. In the reaction mechanism, the compound MnO2 appears. A student makes the claim:
"The order of MnO2 must be zero since it does not appear in the overall balanced equation."
Do you agree or disagree with the student? Explain your reasoning.
6. In trial 1, 10 mL of A and 10 mL of B are mixed and the reaction goes to completion.
i.) Determine the number of moles of B used in the reaction.
ii.) The reaction vessel is heated and all the water is driven off, leaving only 0.124 grams of A2B3(s). Determine the molar mass of A2B3.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Answer questions 1-6 using the information below:
2 A(aq) + 3 B(aq) LaTeX: \longrightarrow⟶ A2B3(aq...
Questions





Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01

Social Studies, 26.06.2020 23:01



Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01

Geography, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01


Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01


Mathematics, 26.06.2020 23:01

Mathematics, 26.06.2020 23:01



