
Chemistry, 11.02.2021 04:10 brooke0713
How many more oxygen atoms are in Na2HPO4H(disodium phosphate) then in 2H3PO(Phosphoric Acid)?
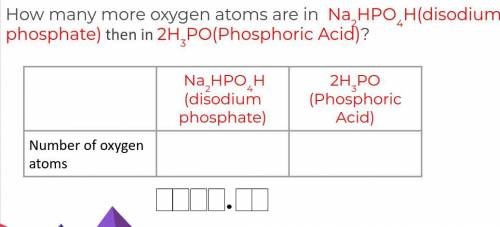

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
How many more oxygen atoms are in Na2HPO4H(disodium phosphate) then in 2H3PO(Phosphoric Acid)?
Questions

Chemistry, 07.10.2020 18:01

Mathematics, 07.10.2020 18:01



Mathematics, 07.10.2020 18:01

Biology, 07.10.2020 18:01


Mathematics, 07.10.2020 18:01

Mathematics, 07.10.2020 18:01


Mathematics, 07.10.2020 18:01

Mathematics, 07.10.2020 18:01


English, 07.10.2020 18:01

Law, 07.10.2020 18:01







