
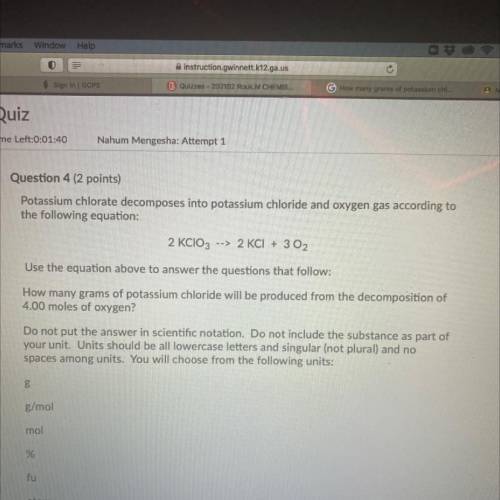
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas according to
the following equation:
2 KClO3 --> 2 KCl + 3 O2
Use the equation above to answer the questions that follow:
How many grams of potassium chloride will be produced from the decomposition of
4.00 moles of oxygen?
Do not put the answer in scientific notation. Do not include the substance as part of
your unit. Units should be all lowercase letters and singular (not plural) and no
spaces among units. You will choose from the following units:
В
g/mol
mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas accordin...
Questions





Mathematics, 09.07.2019 09:30


Chemistry, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30



Geography, 09.07.2019 09:30



English, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30

Social Studies, 09.07.2019 09:30

English, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30


Mathematics, 09.07.2019 09:30



