
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δh∘=−484kj
how much pv work is done in kilojoules for the reaction of 3.20 mol of h2 with 1.60 mol of o2 at atmospheric pressure if the volume change is −22.6l?
express your answer using three significant figures

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1


Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δ...
Questions

Mathematics, 23.02.2020 05:28

Mathematics, 23.02.2020 05:28



History, 23.02.2020 05:29

Advanced Placement (AP), 23.02.2020 05:29

Mathematics, 23.02.2020 05:29

Mathematics, 23.02.2020 05:30




Health, 23.02.2020 05:30


English, 23.02.2020 05:32

Mathematics, 23.02.2020 05:32


Mathematics, 23.02.2020 05:32

Mathematics, 23.02.2020 05:32


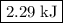 .
.
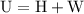
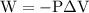
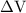 is the change in the volume in liter.
is the change in the volume in liter.
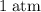 .
.
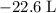 .
.
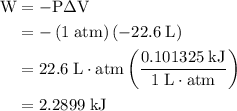
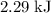 .
.


