
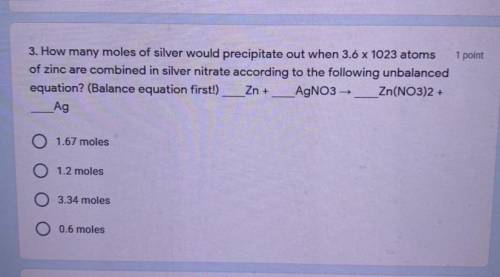
Chemistry, 11.02.2021 14:00 moomoofower
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver nitrate according to the following unbalanced equation? (Balance equation first!)
Zn + AgNO3 → Zn(NO3)2 +Ag
A: 1.67 moles
B: 1.2 moles
C: 3.34 moles
D: 0.6 moles
Can someone please help me with this I don’t get it at all.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

You know the right answer?
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver...
Questions


History, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01




Physics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Social Studies, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

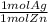 = 0.6 mol Ag
= 0.6 mol Ag

